Graphical and cDFT approaches to the study of fundamental and overtone absorption intensities of some OH stretching vibrations
Masafumi Tsuyuki1, Shunki Furudate1, Yuto Kugaya1, Satoshi Yabushita1
Department of Chemistry, Faculty of Science and Technology, Keio University, 3-14-1 Hiyoshi, Kohoku-ku, Yokohama 223-8522, Japan
In general, the fundamental intensities of OH stretching vibrations depend strongly on the substituents but only weakly on the molecular conformations. Interestingly, completely opposite trends have been seen in the overtone intensities, i.e., only a weak substituent dependence1, but a stronger conformation dependence2. It is well-known that hydrogen bonding increases the OH stretching fundamental intensity, but less well-known is the decrease of the overtone intensities.3 To understand these characteristics in the fundamental and overtone intensities comprehensively, we calculated the OH stretching vibrations (Δ? = 1 − 6) for ethanol and trifluoroethanol (TFE) conformers and hydrogen bonded phenol systems with DFT method in the local mode model.
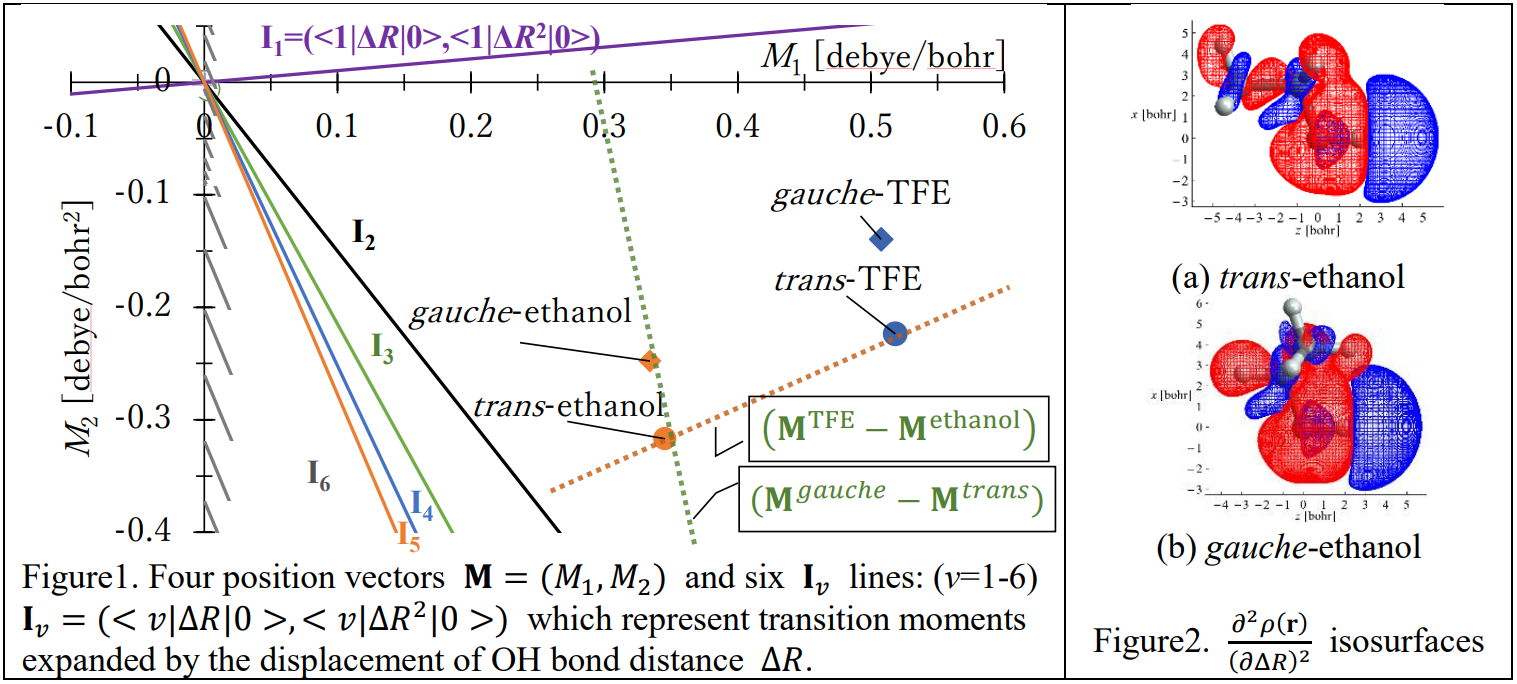
Our calculation showed almost identical fundamental intensities for the trans and gauche isomers, while the former had the larger overtone (Δ? ≥ 2) intensities than the latter. A component of the dipole moment function (vector) of a conformer A of a molecule is expressed as a polynomial of the OH bond displacement ∆R, μA(∆R) = ∑MnA∆Rn
Then, the 0-v transition moment is expressed as the inner product < ?|?A|0 > = ∑?=1 ??A < ?|???|0 > ≡ ?A ⋅ ?? of the two vectors ?A = (?1A, ?2A, ⋯ ) and ?? = (< ?|??|0 >, < ?|??2 |0 >, ⋯ ). This ?? vector is almost independent of the substituents and conformers. Figure 1 shows ?A for each A as scattered points and ?? as lines.4 Since the difference of the transition moments of A and B is < ?|?|0 >A −< ?|?|0 >B= (?A − ?B ) ⋅ ??, the following characteristics are recognized for ? ≥ 2: (1) ?TFE ⋅ ?? = ?ethanol ⋅ ?? because (?TFE − ?ethanol) ⊥ ?? and (2) ?????ℎ? ⋅ ?? ≠ ?????? ⋅ ?? because (?????ℎ? − ??????) ∥ ??.
Since ![]() , the reduced |?2 | going from trans to gauche means that the amount of electron density migration on OH stretching becomes harder. We regarded ?2 as a response of the electron density ?(?) to OH stretching, and calculated
, the reduced |?2 | going from trans to gauche means that the amount of electron density migration on OH stretching becomes harder. We regarded ?2 as a response of the electron density ?(?) to OH stretching, and calculated ![]() following the conceptual DFT.5 Figure 2 shows that |?2 | was reduced because the charge transfer on OH group canceled by that of CH3 group in the gauche isomer. We will discuss the density migration in hydrogen bonded phenol systems in a similar means.
following the conceptual DFT.5 Figure 2 shows that |?2 | was reduced because the charge transfer on OH group canceled by that of CH3 group in the gauche isomer. We will discuss the density migration in hydrogen bonded phenol systems in a similar means.
In summary, the characteristics in the fundamental and overtone intensities described in the first paragraph above was found to be a consequence of the interplay between the mechanical anharmonicity (difference of the ?1and ?? (? ≥ 2) directions), and the electrical anharmonicity represented in the (?1A, ?2A, ⋯ ).
[2] K. Takahashi, M. Sugawara, S. Yabushita, J. Phys. Chem. A, 107 (2003) 11092-11101.
[3] Y. Futami, Y.Ozaki, Phys. Chem. Chem. Phys., 18, (2016) 5580-5586.
[4] H. Takahashi, S. Yabushita, J. Phys. Chem. A., 117 (2013) 5491-5502.
[5] P. Geerlings, S. Fias, Z. Boisdenghien, F. De Proft, Chem. Soc. Rev., 43 (2014) 4989-5008.