Conformational Analysis of Semiconducting Organic Oligomers
Morgan A. Perkins,* Thomas L. Ellington, and Gregory S. Tschumper
Department of Chemistry and Biochemistry, University of Mississippi
University, MS 38677-1848
*Presenting author: mawebb2@go.olemiss.edu
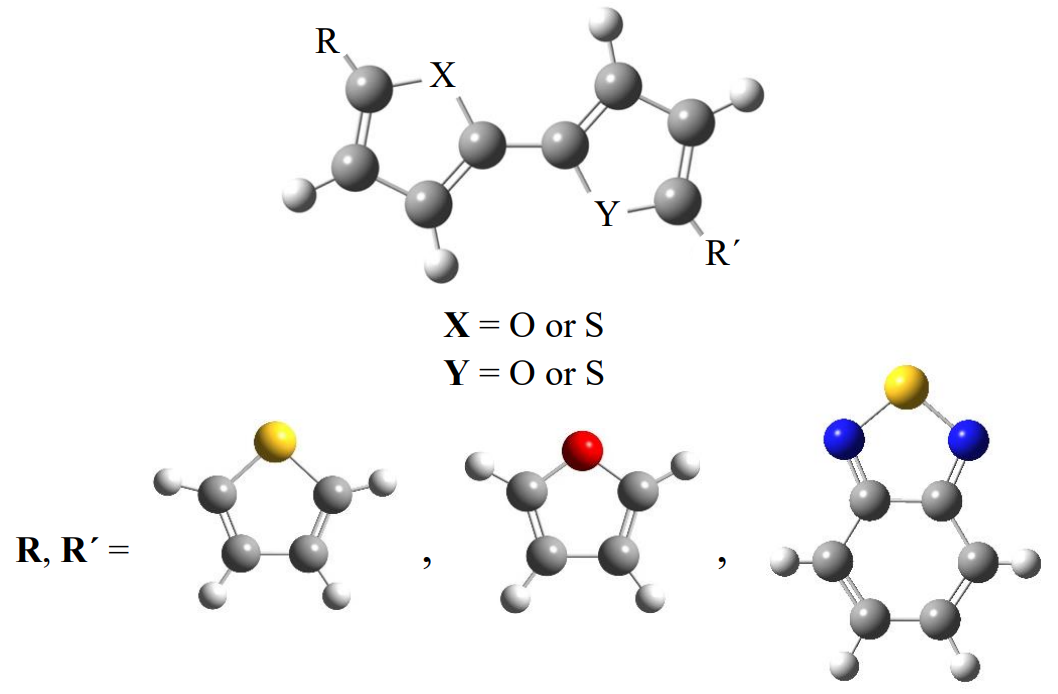
Successful application of organic semiconducting oligomers in electronic devices, such as light emitting diodes (LEDs) or field effect transistors (FETs), depends partly on the oligomer’s rigidity to interconversion and the material’s solid-state packing efficiency [1,2]. In this work, the torsional profiles of furan- and thiophene-containing oligomers are examined with and without the presence of an electron accepting core, such as 2,1,3-benzothiadiazole (BTD). MP2 and CCSD(T) computations on the smaller oligomers are used to calibrate the conformational energetics from some popular density function theory (DFT) methods (e.g., B3LYP, M06-2X, and ωB97XD). These less demanding DFT and MP2 procedures are employed in conjunction with a correlation consistent, triple-ζ basis set (cc-pVTZ) to more thoroughly characterize the smaller systems via full geometry optimizations and harmonic vibrational frequency computations. These methods are also used to carry out torsional scans of a wide range of systems in order to probe the relative energies of various syn and anti configurations (τ(XCCY) ≈ 0° or 180°, respectively, where X,Y = O or S) along with the associated barriers to interconversion.
References
[1] C. Groves. (2017) Rep. Prog. Phys., 80, 026502.
[2] J. Huang, S. Wen, W. Deng, and K. Han. (2011) J. Phys. Chem. B., 2140-2147