Three-Dimensional Master Equation (3DME) Approach
Thanh Lam Nguyen, John Stanton
Quantum Theory Project, Department of Chemistry and Physics, University of Florida,
Gainesville, FL 32611, USA
Email: tlam.nguyen@chem.ufl.edu
The master equation technique is a basic tool to interpret gas-phase experimental results as well as to provide phenomenological rate coefficients (where experimental data are not available) for modeling. For some reactions (for example, dissociations through a loose, variational TS such as C2H6 → CH3 + CH3, O3 → O2 + O, NH3 → NH2 + H, so on) where there are significant changes of rotational constants along the reaction coordinate from a reactant via a transition state to product(s), including effects of angular momentum explicitly into a master equation model is a necessity. While the total angular momentum that corresponds to the quantum number J is always conserved, its projection (the K quantum number) is not. Therefore, the convolution of external rotational quantum states with vibrational quantum states depends on how K is treated: as either adiabatic or active. Because of these considerations, there are, in principle, four different models that can be used to compute microcanonical rate constants:1 (i) first, model-I assumes that K is active for both TS and reactant; (ii) second, model-II uses K adiabatic for both TS and reactant; (iii) third, model-III applies K active to TS, but K adiabatic to reactant; and (iv) final, model-IV assigns K adiabatic to TS, but K active to reactant.
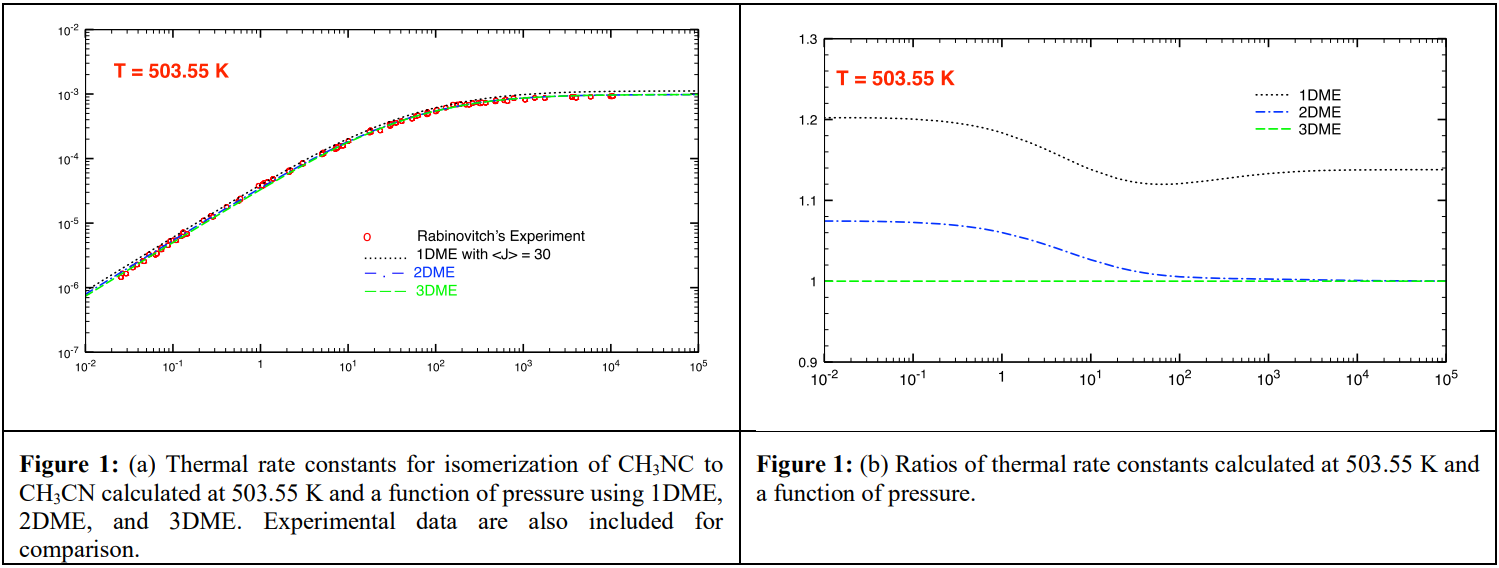
Model-I will result in a two-dimensional master equation (2DME) approach, whose solutions have been reported (by us) recently.2-4 The remaining models will lead to a three-dimensional master equation, whose solutions has recently been given for the first time.5 In that work, we developed an algorithm for solutions of 3DME using model-II. Two examples (one is for a thermally activated isomerization of CH3NC to CH3CN6 via a tight TS (see Figure 1 below); and the other is for a thermally activated dissociation of NH3 to H + NH2 via a loose, variational TS) will be discussed. In addition, comparison of 3DME results with those of 1DME and 2DME will also be presented.
1. Zhu, L.; Chen, W.; Hase, W. L.; Kaiser, E. W., Comparison of Models for Treating Angular-Momentum in Rrkm Calculations with Vibrator Transition-States – Pressure and Temperature-Dependence of Cl+C2h2 Association. J Phys Chem-Us 1993, 97 (2), 311-322.
2. Nguyen, T. L.; Stanton, J. F., A Steady-State Approximation to the Two-Dimensional Master Equation for Chemical Kinetics Calculations. J Phys Chem A 2015, 119 (28), 7627-7636.
3. Nguyen, T. L.; Lee, H.; Matthews, D. A.; McCarthy, M. C.; Stanton, J. F., Stabilization of the Simplest Criegee Intermediate from the Reaction between Ozone and Ethylene: A High-Level Quantum Chemical and Kinetic Analysis of Ozonolysis. J Phys Chem A 2015, 119 (22), 5524-5533.
4. Nguyen, T. L.; McCaslin, L.; McCarthy, M. C.; Stanton, J. F., Communication: Thermal unimolecular decomposition of synCH3CHOO: A kinetic study. J Chem Phys 2016, 145 (13), 131102.
5. Nguyen, T. L.; Stanton, J. F., Three-Dimensional Master Equation (3DME) Approach. J Phys Chem A 2018, 122 (38), 7757-7767.
6. Nguyen, T. L.; Thorpe, J. H.; Bross, D. H.; Ruscic, B.; Stanton, J. F., Unimolecular Reaction of Methyl Isocyanide to Acetonitrile: A High-Level Theoretical Study. J Phys Chem Lett 2018, 9 (10), 2532-2538.