Macroscopic pKa Prediction of Polyprotic Acids from DFT Calculations: The Case of Acetohydroxamic Acid
Rodrigo Casasnovas,1 Juan Frau,1 Nelaine Mora-Díez2*
1Universitat de les Illes Balears, Departament de Química, Palma de Mallorca, Spain
2Thompson Rivers University, Department of Chemistry, Kamloops,
Canada Email: nmora@tru.ca
The accurate calculation of pKa values in solution is a current challenge in computational chem- istry and many computational approaches have been proposed to tackle this problem. In particu- lar, it is especially difficult to estimate macroscopic pKa values of polyprotic acids from DFT- derived microscopic pKa values due to the errors of the latter. The goal of this study is to account for the existence of all the acid-base equilibria in acetohydroxamic acid (AHA) and come up with the methodology that best yields its macroscopic pKa in water. DFT calculations were com- bined with pure continuum and cluster-continuum approaches, which were used to model the solvent effects.
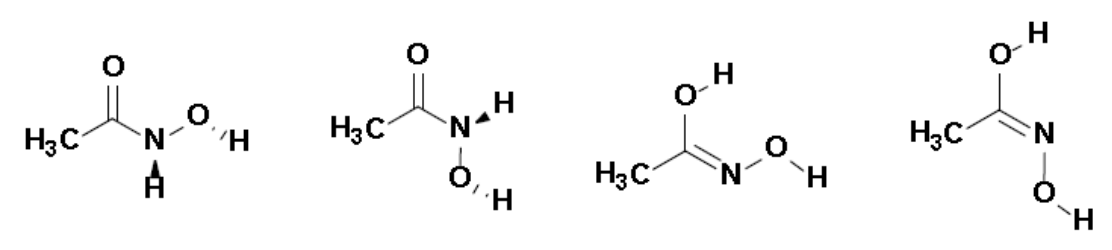
AHA is a drug used with antibiotics and/or surgery to treat certain types of bladder infections. It is also used as a chelating ligand for organometallic compounds. AHA exists in an equilibrium involving four main isomers (the amide and imide forms in their Z and E conformations, see Figure 1) each of which possesses two acid sites. Hence, each neutral form can dissociate in aqueous solution and exists in equilibrium with its corresponding anionic forms. These multiple dissociation equilibria contribute to the relatively low overall pKa of this compound.